Mucopolysaccharidosis Type VI, an Updated Overview of the Disease
Abstract
:1. Introduction
2. Epidemiology
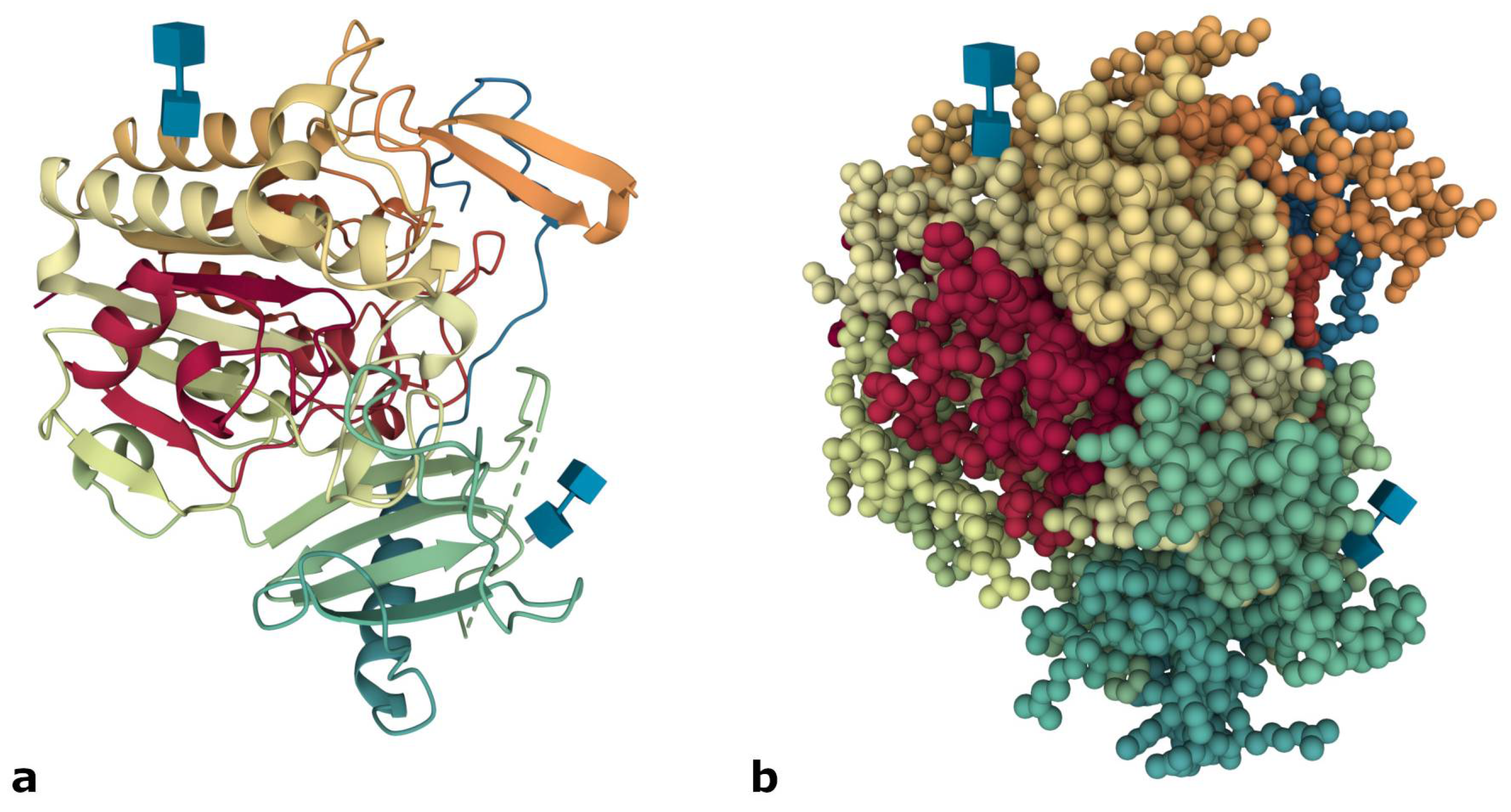
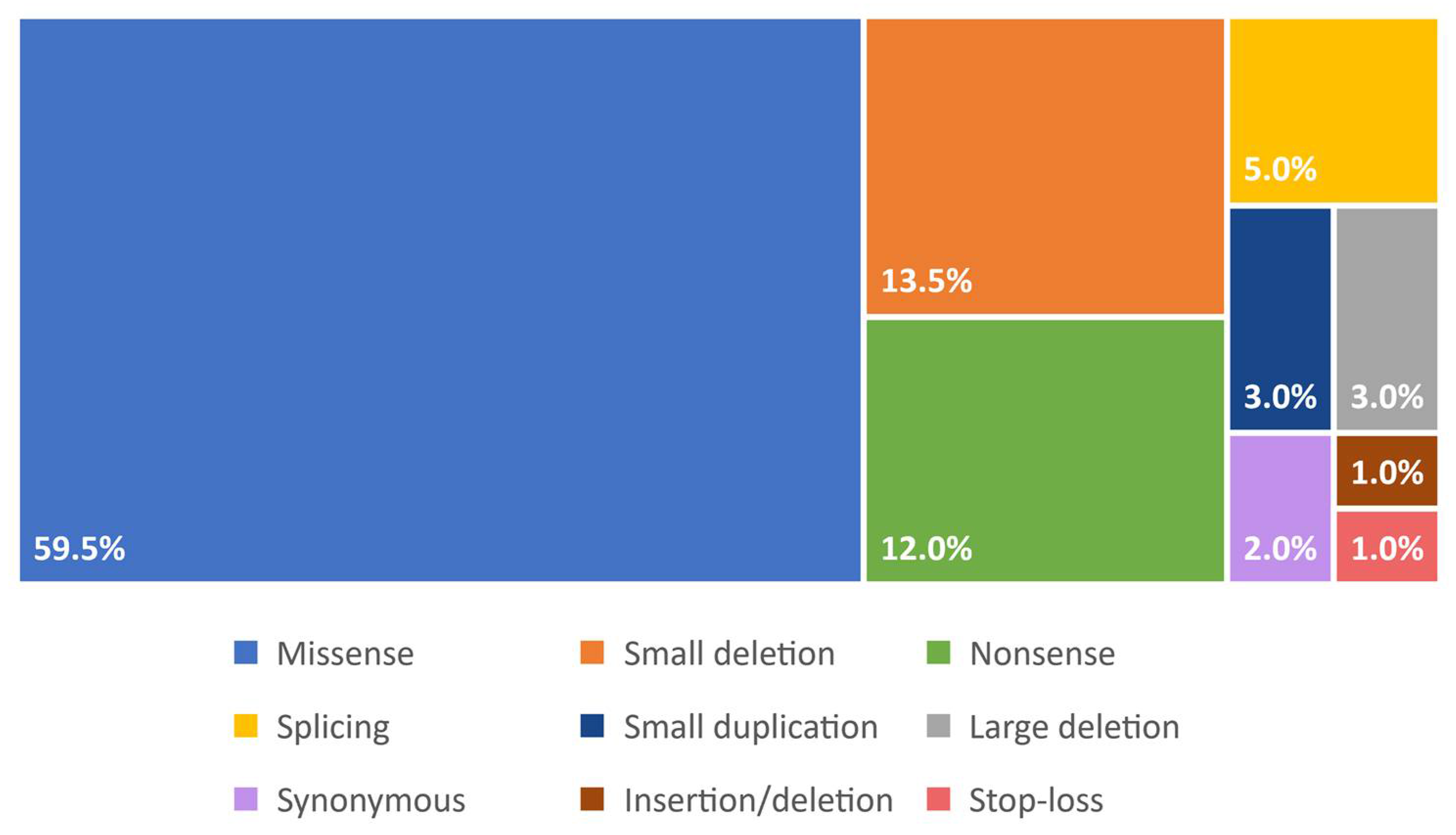
3. Molecular Basis
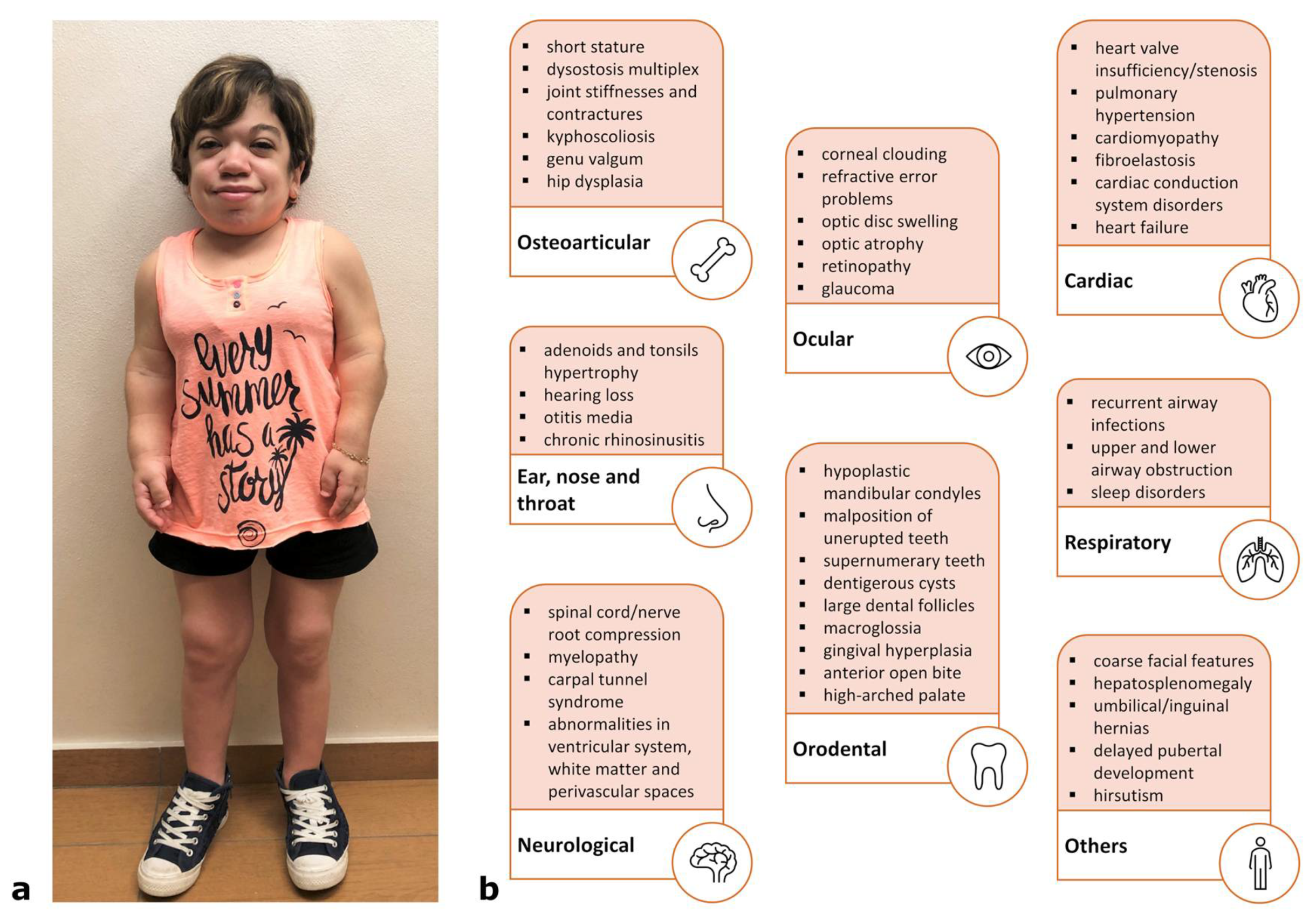
4. Clinical Features and Degrees of Severity
4.1. Rapidly Progressing Forms
4.2. Slowly Progressing Forms
5. Diagnosis
Newborn Screening (NBS)
6. Treatment
6.1. Management of Symptoms
6.2. Enzyme Replacement Therapy (ERT)
Intra-Articular ERT
6.3. Bone Marrow Transplantation (BMT) and Hematopoietic Stem Cells Transplantation (HSCT)
6.4. Other Therapies
6.4.1. Gene Therapy
6.4.2. Substrate Reduction Therapy
6.4.3. Anti-Inflammatory Drugs
6.4.4. Stop Codon Read-Through
7. Experimental Models and Pathogenesis
7.1. In Vitro Studies
7.2. Animal Models
7.2.1. Feline Spontaneous Model
7.2.2. Rat Spontaneous Model
7.2.3. Mouse Models
7.2.4. Dog Spontaneous Mutants
8. Biomarkers
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harmatz, P.R.; Shediac, R. Mucopolysaccharidosis VI: Pathophysiology, diagnosis and treatment. Front. Biosci. Landmark 2017, 22, 385–406. [Google Scholar] [CrossRef] [Green Version]
- Maroteaux, P.; Leveque, B.; Marie, J.; Lamy, M. A new dysostosis with urinary elimination of chondroitin sulfate B. Presse Med. 1963, 71, 1849–1852. [Google Scholar]
- Baron, R.W.; Neufeld, E.F. A distinct biochemical deficit in the Maroteaux-Lamy syndrome (mucopolysaccharidosis VI). J. Pediatr. 1972, 80, 114–116. [Google Scholar] [CrossRef]
- Jurecka, A.; Ługowska, A.; Golda, A.; Czartoryska, B.; Tylki-Szymańska, A. Prevalence rates of mucopolysaccharidoses in Poland. J. Appl. Genet. 2015, 56, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Moammar, H.; Cheriyan, G.; Mathew, R.; Al-Sannaa, N. Incidence and patterns of inborn errors of metabolism in the Eastern Province of Saudi Arabia, 1983–2008. Ann. Saudi Med. 2010, 30, 271. [Google Scholar] [CrossRef] [Green Version]
- Costa-Motta, F.M.; Bender, F.; Acosta, A.; Abé-Sandes, K.; Machado, T.; Bomfim, T.; Sorte, T.B.; Da Silva, D.; Bittles, A.; Giugliani, R.; et al. A community-based study of mucopolysaccharidosis type VI in Brazil: The influence of founder effect, endogamy and consanguinity. Hum. Hered. 2014, 77, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagnostics 2021, 11, 273. [Google Scholar] [CrossRef]
- Chan, M.J.; Liao, H.C.; Gelb, M.H.; Chuang, C.K.; Liu, M.Y.; Chen, H.J.; Kao, S.M.; Lin, H.Y.; Huang, Y.H.; Kumar, A.B.; et al. Taiwan National Newborn Screening Program by Tandem Mass Spectrometry for Mucopolysaccharidoses Types I, II, and VI. J. Pediatr. 2019, 205, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Lee, N.C.; Chen, P.W.; Yeh, H.Y.; Gelb, M.H.; Chiu, P.C.; Chu, S.Y.; Lee, C.H.; Lee, A.R.; Hwu, W.L. Newborn screening for Morquio disease and other lysosomal storage diseases: Results from the 8-plex assay for 70,000 newborns. Orphanet J. Rare Dis. 2020, 15, 38. [Google Scholar] [CrossRef]
- Scott, C.R.; Elliott, S.; Hong, X.; Huang, J.Y.; Kumar, A.B.; Yi, F.; Pendem, N.; Chennamaneni, N.K.; Gelb, M.H. Newborn Screening for Mucopolysaccharidoses: Results of a Pilot Study with 100,000 Dried Blood Spots. J. Pediatr. 2020, 216, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.S.; Clements, P.R.; Ashby, S.J.; Collyer, C.A.; Harrop, S.J.; Hopwood, J.J.; Guss, J.M. Structure of a human lysosomal sulfatase. Structure 1997, 5, 277–289. [Google Scholar] [CrossRef]
- Saito, S.; Ohno, K.; Sugawara, K.; Sakuraba, H. Structural and clinical implications of amino acid substitutions in N-acetylgalactosamine-4-sulfatase: Insight into mucopolysaccharidosis type VI. Mol. Genet. Metab. 2008, 93, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J.; Bertozzi, C.R. Formylglycine, a post-translationally generated residue with unique catalytic capabilities and biotechnology applications. ACS Chem. Biol. 2015, 10, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Tomanin, R.; Karageorgos, L.; Zanetti, A.; Al-Sayed, M.; Bailey, M.; Miller, N.; Sakuraba, H.; Hopwood, J.J. Mucopolysaccharidosis type VI (MPS VI) and molecular analysis: Review and classification of published variants in the ARSB gene. Hum. Mutat. 2018, 39, 1788–1802. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, S.; Noruzinia, M.; Bashti, O.; Ahmadvand, M.; Salehi Chaleshtori, A.R.; Mahootipou, L. Another Novel Missense Mutation in ARSB Gene in Iran. Acta Med. Iran. 2017, 55, 585–590. [Google Scholar]
- Al-Sannaa, N.A.; Al-Abdulwahed, H.Y.; Al-Majed, S.I.; Bouholaigah, I.H. The clinical and genetic Spectrum of Maroteaux-Lamy syndrome (Mucopolysaccharidosis VI) in the Eastern Province of Saudi Arabia. J. Community Genet. 2018, 9, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Pinto e Vairo, F.; Conboy, E.; de Souza, C.F.M.; Jones, A.; Barnett, S.S.; Klee, E.W.; Lanpher, B.C. Diagnosis of Attenuated Mucopolysaccharidosis VI: Clinical, Biochemical, and Genetic Pitfalls. Pediatrics 2018, 142, e20180658. [Google Scholar] [CrossRef] [Green Version]
- Zapała, B.; Chmura, O.; Ciałowicz, U.; Solnica, B.; Krajewska-Włodarczyk, M.; Żuber, Z. A case of mucopolysaccharidosis type VI in a polish family. Importance of genetic testing and genotype-phenotype relationship in the diagnosis of mucopolysaccharidosis. Mol. Genet. Metab. Rep. 2020, 25, 100658. [Google Scholar] [CrossRef]
- Aminzadeh, M.; Malekpour, N.; Ghandil, P. Identification of arylsulfatase B gene mutations and clinical presentations of Iranian patients with Mucopolysaccharidosis VI. Gene 2019, 706, 1–5. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Encarnação, M.; Matos, L.; Silva, L.; Ribeiro, D.; Santos, J.I.; João Prata, M.; Vilarinho, L.; Alves, S. Molecular Characterization of a Novel Splicing Mutation underlying Mucopolysaccharidosis (MPS) type VI-Indirect Proof of Principle on Its Pathogenicity. Diagnostics 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Hançer, V.S.; Büyükdoǧan, M.; Babameto-Laku, A. A Novel Pathological ARSB Mutation (c.870G>A; p.Trp290stop) in Mucopolysaccharidosis Type VI Patients. Mol. Syndromol. 2020, 10, 272–275. [Google Scholar] [CrossRef]
- He, M.F.; Yang, J.; Dong, M.J.; Wang, Y.T.; Liu, H. Compound heterozygous missense mutations in a Chinese mucopolysaccharidosis type VI patient: A case report. BMC Ophthalmol. 2021, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Jafaryazdi, R.; Shams, S.; Isaian, A.; Setoodeh, A.; Teimourian, S. Identification of eleven different mutations including six novel, in the arylsulfatase B gene in Iranian patients with mucopolysaccharidosis type VI. Mol. Biol. Rep. 2019, 46, 3417–3426. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, M.; Dursun, A.; Coşkun, T.; Tokatlı, A.; Özgül, R.K.; Yücel-Yılmaz, D.; Karaca, M.; Doğru, D.; Alehan, D.; Kadayıfçılar, S.; et al. Genotypic-phenotypic features and enzyme replacement therapy outcome in patients with mucopolysaccharidosis VI from Turkey. Am. J. Med. Genet. A 2017, 173, 2954–2967. [Google Scholar] [CrossRef]
- Ley-Martos, M.; Guerrero, J.M.; Lucas-Javato, M.; Remón-García, C.; Raul García-Lozano, J.; Colón, C.; Crujeiras, P.; Rodrigues, D.; Paul-Sánchez, P.; Macher, H.C. Family study of a novel mutation of mucopolysaccharidosis type VI with a severe phenotype and good response to enzymatic replacement therapy: Case report. Medicine 2018, 97, e12872. [Google Scholar] [CrossRef]
- Malekpour, N.; Vakili, R.; Hamzehloie, T. Mutational analysis of ARSB gene in mucopolysaccharidosis type VI: Identification of three novel mutations in Iranian patients. Iran. J. Basic Med. Sci. 2018, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne-Weiler, T.; Howard, J.; Mort, M.; Cooper, D.N.; Sanford, J.R. Loss of exon identity is a common mechanism of human inherited disease. Genome Res. 2011, 21, 1563–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broeders, M.; Smits, K.; Goynuk, B.; Oussoren, E.; van den Hout, H.J.M.P.; Bergsma, A.J.; van der Ploeg, A.T.; Pijnappel, W.W.M.P. A Generic Assay to Detect Aberrant ARSB Splicing and mRNA Degradation for the Molecular Diagnosis of MPS VI. Mol. Ther. Methods Clin. Dev. 2020, 19, 174–185. [Google Scholar] [CrossRef]
- Marek-Yagel, D.; Eliyahu, A.; Veber, A.; Shalva, N.; Philosoph, A.M.; Barel, O.; Javasky, E.; Pode-Shakked, B.; Loewenthal, N.; Anikster, Y.; et al. Deep intronic variant in the ARSB gene as the genetic cause for Maroteaux-Lamy syndrome (MPS VI). Am. J. Med. Genet. A 2021, 185, 3804–3809. [Google Scholar] [CrossRef]
- Nicolas-Jilwan, M.; AlSayed, M. Mucopolysaccharidoses: Overview of neuroimaging manifestations. Pediatr. Radiol. 2018, 48, 1503–1520. [Google Scholar] [CrossRef]
- Kantaputra, P.N.; Kayserili, H.; Güven, Y.; Kantaputra, W.; Balci, M.C.; Tanpaiboon, P.; Uttarilli, A.; Dalal, A. Oral manifestations of 17 patients affected with mucopolysaccharidosis type VI. J. Inherit. Metab. Dis. 2014, 37, 263–268. [Google Scholar] [CrossRef]
- Ballıkaya, E.; Eymirli, P.S.; Yıldız, Y.; Avcu, N.; Sivri, H.S.; Uzamış-Tekçiçek, M. Oral health status in patients with mucopolysaccharidoses. Turk. J. Pediatr. 2018, 60, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, E.G.; Eisengart, J.B. The natural history of neurocognition in MPS disorders: A review. Mol. Genet. Metab. 2021, 133, 8–34. [Google Scholar] [CrossRef] [PubMed]
- Ebbink, B.J.; Brands, M.M.G.; van den Hout, J.M.P.; Lequin, M.H.; van den Braak, R.R.J.C.; van de Weitgraven, R.L.; Plug, I.; Aarsen, F.K.; van der Ploeg, A.T. Long-term cognitive follow-up in children treated for Maroteaux-Lamy syndrome. J. Inherit. Metab. Dis. 2016, 39, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Shapiro, E.; Rudser, K.; Kunin-Batson, A.; King, K.; Whitley, C.B. Association of somatic burden of disease with age and neuropsychological measures in attenuated mucopolysaccharidosis types I, II and VI. Mol. Genet. Metab. Rep. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Isbrandt, D.; Arlt, G.; Brooks, D.A.; Hopwood, J.J.; Von Figura, K.; Peters, C. Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): Six unique arylsulfatase B gene alleles causing variable disease phenotypes. Am. J. Hum. Genet. 1994, 54, 454. [Google Scholar]
- Neufeld, E.F.; Muenzer, J. The mucopolysaccharidoses. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Childs, B., Kinzler, K.W., Vogelstein, B., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Quartel, A.; Hendriksz, C.J.; Parini, R.; Graham, S.; Lin, P.; Harmatz, P. Growth Charts for Individuals with Mucopolysaccharidosis VI (Maroteaux-Lamy Syndrome). JIMD Rep. 2015, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiedler, S.J.; Beck, M.; Bajbouj, M.; Giugliani, R.; Schwartz, I.; Harmatz, P.; Wraith, J.E.; Roberts, J.; Ketteridge, D.; Hopwood, J.J.; et al. Threshold effect of urinary glycosaminoglycans and the walk test as indicators of disease progression in a survey of subjects with Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Am. J. Med. Genet. A 2005, 134A, 144–150. [Google Scholar] [CrossRef]
- Valayannopoulos, V.; Nicely, H.; Harmatz, P.; Turbeville, S. Mucopolysaccharidosis VI. Orphanet J. Rare Dis. 2010, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Oussoren, E.; Bessems, J.H.J.M.; Pollet, V.; van der Meijden, J.C.; van der Giessen, L.J.; Plug, I.; Devos, A.S.; Ruijter, G.J.G.; van der Ploeg, A.T.; Langeveld, M. A long term follow-up study of the development of hip disease in Mucopolysaccharidosis type VI. Mol. Genet. Metab. 2017, 121, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.E.; Howell, R.R.; McKusick, V.A. Corneal clouding in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Birth Defects Orig Artic Ser. 1971, 7, 156–158. [Google Scholar]
- Ashworth, J.L.; Biswas, S.; Wraith, E.; Lloyd, I.C. The ocular features of the mucopolysaccharidoses. Eye 2006, 20, 553–563. [Google Scholar] [CrossRef]
- Ferrari, S.; Ponzin, D.; Ashworth, J.L.; Fahnehjelm, K.T.; Summers, C.G.; Harmatz, P.R.; Scarpa, M. Diagnosis and management of ophthalmological features in patients with mucopolysaccharidosis. Br. J. Ophthalmol. 2011, 95, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Decker, C.; Yu, Z.F.; Giugliani, R.; Schwartz, I.V.D.; Guffon, N.; Teles, E.L.; Miranda, M.C.S.; Wraith, J.E.; Beck, M.; Arash, L.; et al. Enzyme replacement therapy for mucopolysaccharidosis VI: Growth and pubertal development in patients treated with recombinant human N-acetylgalactosamine 4-sulfatase. J. Pediatr. Rehabil. Med. 2010, 3, 89–100. [Google Scholar] [CrossRef]
- Giugliani, R.; Lampe, C.; Guffon, N.; Ketteridge, D.; Leão-Teles, E.; Wraith, J.E.; Jones, S.A.; Piscia-Nichols, C.; Lin, P.; Quartel, A.; et al. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)-10-year follow-up of patients who previously participated in an MPS VI survey study. Am. J. Med. Genet. Part. A 2014, 164, 1953–1964. [Google Scholar] [CrossRef] [Green Version]
- Jurecka, A.; Zakharova, E.; Malinova, V.; Voskoboeva, E.; Tylki-Szymańska, A. Attenuated osteoarticular phenotype of type VI mucopolysaccharidosis: A report of four patients and a review of the literature. Clin. Rheumatol. 2014, 33, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, D.A.; Gibson, G.J.; Karageorgos, L.; Hein, L.K.; Robertson, E.F.; Hopwood, J.J. An index case for the attenuated end of the mucopolysaccharidosis type VI clinical spectrum. Mol. Genet. Metab. 2005, 85, 236–238. [Google Scholar] [CrossRef]
- Scarpa, M.; Buffone, E.; La Marca, P.L.; Campello, M.; Rampazzo, A. Difficulties in diagnosing slowly progressive mucopolysaccharidosis VI: A case series. J. Pediatr. Rehabil. Med. 2010, 3, 71–75. [Google Scholar] [CrossRef]
- Gottwald, I.; Hughes, J.; Stewart, F.; Tylee, K.; Church, H.; Jones, S.A. Attenuated mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome) due to homozygosity for the p.Y210C mutation in the ARSB gene. Mol. Genet. Metab. 2011, 103, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Thümler, A.; Miebach, E.; Lampe, C.; Pitz, S.; Kamin, W.; Kampmann, C.; Link, B.; Mengel, E. Clinical characteristics of adults with slowly progressing mucopolysaccharidosis VI: A case series. J. Inherit. Metab. Dis. 2012, 35, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Hendriksz, C.J.; Giugliani, R.; Harmatz, P.; Lampe, C.; Martins, A.M.; Pastores, G.M.; Steiner, R.D.; Leão Teles, E.; Valayannopoulos, V. Design, baseline characteristics, and early findings of the MPS VI (mucopolysaccharidosis VI) Clinical Surveillance Program (CSP). J. Inherit. Metab. Dis. 2013, 36, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mason, R.W.; Kobayashi, H.; Yamaguchi, S.; Tomatsu, S. Advances in glycosaminoglycan detection. Mol. Genet. Metab. 2020, 130, 101–109. [Google Scholar] [CrossRef]
- Filocamo, M.; Tomanin, R.; Bertola, F.; Morrone, A. Biochemical and molecular analysis in mucopolysaccharidoses: What a paediatrician must know. Ital. J. Pediatr. 2018, 44. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.; Bodamer, O.A.; Burin, M.G.; D’Almeida, V.; Fietz, M.; Giugliani, R.; Hawley, S.M.; Hendriksz, C.J.; Hwu, W.L.; Ketteridge, D.; et al. Expert recommendations for the laboratory diagnosis of MPS VI. Mol. Genet. Metab. 2012, 106, 73–82. [Google Scholar] [CrossRef]
- Akyol, M.U.; Alden, T.D.; Amartino, H.; Ashworth, J.; Belani, K.; Berger, K.I.; Borgo, A.; Braunlin, E.; Eto, Y.; Gold, J.I.; et al. Recommendations for the management of MPS VI: Systematic evidence- and consensus-based guidance. Orphanet J. Rare Dis. 2019, 14. [Google Scholar] [CrossRef]
- Demis, A.A.; Oikonomidou, S.; Daglis, F.; Polymenakos, S.; Panagiotou, M. Double valve replacement in a patient with Maroteaux—Lamy syndrome as an ultimate team challenge. J. Cardiothorac. Surg. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Biomarin. Naglazyme® (Galsulfase) for MPS VI. Available online: https://www.biomarin.com/our-treatments/products/naglazyme-galsulfase-for-mps-vi/ (accessed on 28 September 2021).
- Anson, D.S.; Taylor, J.A.; Bielicki, J.; Harper, G.S.; Peters, C.; Gibson, G.J.; Hopwood, J.J. Correction of human mucopolysaccharidosis type-VI fibroblastsWith recombinant N-acetylgalactosamine-4-sulphatase. Biochem. J. 1992, 284, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezyk, P.F.; Haskins, M.E.; Patterson, D.F.; Mellman, W.J.; Greenstein, M. Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: A model of Maroteaux-Lamy syndrome. Science 1977, 198, 834–836. [Google Scholar] [CrossRef]
- Haskins, M.E.; Aguirre, G.D.; Jezyk, P.F.; Patterson, D.F. The pathology of the feline model of mucopolysaccharidosis VI. Am. J. Pathol. 1980, 101, 657–674. [Google Scholar]
- Crawley, A.C.; Brooks, D.A.; Muller, V.J.; Petersen, B.A.; Isaac, E.L.; Bielicki, J.; King, B.M.; Boulter, C.D.; Moore, A.J.; Fazzalari, N.L.; et al. Enzyme replacement therapy in a feline model of Maroteaux-Lamy syndrome. J. Clin. Investig. 1996, 97, 1864–1873. [Google Scholar] [CrossRef] [Green Version]
- Crawley, A.C.; Niedzielski, K.H.; Isaac, E.L.; Davey, R.C.A.; Byers, S.; Hopwood, J.J. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J. Clin. Investig. 1997, 99, 651–662. [Google Scholar] [CrossRef]
- Bielicki, J.; Crawley, A.C.; Davey, R.C.; Varnai, J.C.; Hopwood, J.J. Advantages of using same species enzyme for replacement therapy in a feline model of mucopolysaccharidosis type VI. J. Biol. Chem. 1999, 274, 36335–36343. [Google Scholar] [CrossRef] [Green Version]
- Byers, S.; Nuttall, J.D.; Crawley, A.C.; Hopwood, J.J.; Smith, K.; Fazzalari, N.L. Effect of Enzyme Replacement Therapy on Bone Formation in a Feline Model. of Mucopolysaccharidosis Type VI. Bone 1997, 21, 425–431. [Google Scholar] [CrossRef]
- Auclair, D.; Hopwood, J.J.; Brooks, D.A.; Lemontt, J.F.; Crawley, A.C. Replacement therapy in Mucopolysaccharidosis type VI: Advantages of early onset of therapy. Mol. Genet. Metab. 2003, 78, 163–174. [Google Scholar] [CrossRef]
- Harmatz, P.; Whitley, C.B.; Waber, L.; Pais, R.; Steiner, R.; Plecko, B.; Kaplan, P.; Simon, J.; Butensky, E.; Hopwood, J.J. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J. Pediatr. 2004, 144, 574–580. [Google Scholar] [CrossRef]
- Harmatz, P.; Ketteridge, D.; Giugliani, R.; Guffon, N.; Teles, E.L.; Miranda, M.C.S.; Yu, Z.F.; Swiedler, S.J.; Hopwood, J.J. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): Results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylga. Pediatrics 2005, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmatz, P.; Giugliani, R.; Schwartz, I.; Guffon, N.; Teles, E.L.; Miranda, M.C.S.; Wraith, J.E.; Beck, M.; Arash, L.; Scarpa, M.; et al. Enzyme replacement therapy for mucopolysaccharidosis VI: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-l. J. Pediatr. 2006, 148. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P.; Giugliani, R.; Ida, I.V.; Guffon, N.; Teles, E.L.; Miranda, M.C.S.; Wraith, J.E.; Beck, M.; Arash, L.; Scarpa, M.; et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol. Genet. Metab. 2008, 94, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Horovitz, D.D.G.; Leão, E.K.E.A.; Ribeiro, E.M.; Martins, A.M.; Barth, A.L.; Neri, J.I.C.F.; Kerstenetzky, M.; Siqueira, A.C.M.; Ribeiro, B.F.R.; Kim, C.A.; et al. Long-term impact of early initiation of enzyme replacement therapy in 34 MPS VI patients: A resurvey study. Mol. Genet. Metab. 2021, 133, 94–99. [Google Scholar] [CrossRef]
- Harmatz, P.R.; Garcia, P.; Guffon, N.; Randolph, L.M.; Shediac, R.; Braunlin, E.; Lachman, R.S.; Decker, C. Galsulfase (Naglazyme®) therapy in infants with mucopolysaccharidosis VI. J. Inherit. Metab. Dis. 2014, 37, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.; Phillips, D.; Johnson, J.A.; Martin, K.; Randolph, L.M.; Rosenfeld, H.; Harmatz, P. Long-term outcomes of patients with mucopolysaccharidosis VI treated with galsulfase enzyme replacement therapy since infancy. Mol. Genet. Metab. 2021, 133, 100–108. [Google Scholar] [CrossRef]
- McGill, J.J.; Inwood, A.C.; Coman, D.J.; Lipke, M.L.; de Lore, D.; Swiedler, S.; Hopwood, J.J. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age-a sibling control study. Clin. Genet. 2010, 77, 492–498. [Google Scholar] [CrossRef]
- Franco, J.F.; Soares, D.C.; Torres, L.C.; Leal, G.N.; Cunha, M.T.; Honjo, R.S.; Bertola, D.R.; Kim, C.A. Impact of early enzyme-replacement therapy for mucopolysaccharidosis VI: Results of a long-term follow-up of Brazilian siblings. Genet. Mol. Res. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Furujo, M.; Kubo, T.; Kosuga, M.; Okuyama, T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI. Mol. Genet. Metab. 2011, 104, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Furujo, M.; Kosuga, M.; Okuyama, T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI: 10-Year follow up. Mol. Genet. Metab. Rep. 2017, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P.; Hendriksz, C.J.; Lampe, C.; McGill, J.J.; Parini, R.; Leão-Teles, E.; Valayannopoulos, V.; Cole, T.J.; Matousek, R.; Graham, S.; et al. The effect of galsulfase enzyme replacement therapy on the growth of patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Mol. Genet. Metab. 2017, 122, 107–112. [Google Scholar] [CrossRef]
- Lampe, C.; Harmatz, P.R.; Parini, R.; Sharma, R.; Teles, E.L.; Johnson, J.; Sivam, D.; Sisic, Z. Enzyme replacement therapy initiated in adulthood: Findings from the mucopolysaccharidosis VI Clinical Surveillance Program. Mol. Genet. Metab. 2019, 127, 355–360. [Google Scholar] [CrossRef]
- Harmatz, P.R.; Lampe, C.; Parini, R.; Sharma, R.; Teles, E.L.; Johnson, J.; Sivam, D.; Sisic, Z. Enzyme replacement therapy outcomes across the disease spectrum: Findings from the mucopolysaccharidosis VI Clinical Surveillance Program. J. Inherit. Metab. Dis. 2019, 42, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, D.F.; Gallo, L.G.; Leite, B.F.; Silva, R.B.; da Silva, E.N. Clinical effectiveness of enzyme replacement therapy with galsulfase in mucopolysaccharidosis type VI treatment: Systematic review. J. Inherit. Metab. Dis. 2019, 42, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Auclair, D.; Hein, L.K.; Hopwood, J.J.; Byers, S. Intra-articular enzyme administration for joint disease in feline mucopolysaccharidosis VI: Enzyme dose and interval. Pediatr. Res. 2006, 59, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auclair, D.; Hopwood, J.J.; Lemontt, J.F.; Chen, L.; Byers, S. Long-term intra-articular administration of recombinant human N-acetylgalactosamine-4-sulfatase in feline mucopolysaccharidosis VI. Mol. Genet. Metab. 2007, 91, 352–361. [Google Scholar] [CrossRef]
- Auclair, D.; Ketteridge, D.; Oates, S.; Hopwood, J.J.; Byers, S. An overview of intra-articular therapy for mucopolysaccharidosis VI. J. Pediatr. Rehabil. Med. 2010, 3, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Turbeville, S.; Nicely, H.; Rizzo, J.D.; Pedersen, T.L.; Orchard, P.J.; Horwitz, M.E.; Horwitz, E.M.; Veys, P.; Bonfim, C.; Al-Seraihy, A. Clinical outcomes following hematopoietic stem cell transplantation for the treatment of mucopolysaccharidosis VI. Mol. Genet. Metab. 2011, 102, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Sillence, D.; Waters, K.; Donaldson, S.; Shaw, P.J.; Ellaway, C. Combined Enzyme Replacement Therapy and Hematopoietic Stem Cell Transplantation in Mucopolysacharidosis Type VI. JIMD Rep. 2012, 2, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Sohn, Y.; Park, S.; Kim, S.; Cho, S.; Ji, S.; Kwon, E.; Han, S.; Oh, S.; Park, Y.; Ko, A.; et al. Enzyme replacement therapy improves joint motion and outcome of the 12-min walk test in a mucopolysaccharidosis type VI patient previously treated with bone marrow transplantation. Am. J. Med. Genet. A 2012, 158A, 1158–1163. [Google Scholar] [CrossRef]
- Yogalingam, G.; Muller, V.; Hopwood, J.J.; Anson, D.S. Regulation of N-acetylgalactosamine 4-sulfatase expression in retrovirus-transduced feline mucopolysaccharidosis type VI muscle cells. DNA Cell Biol. 1999, 18, 187–195. [Google Scholar] [CrossRef]
- Ponder, K.P.; O’Malley, T.M.; Wang, P.; O’Donnell, P.A.; Traas, A.M.; Knox, V.W.; Aguirre, G.A.; Ellinwood, N.M.; Metcalf, J.A.; Wang, B.; et al. Neonatal gene therapy with a gamma retroviral vector in mucopolysaccharidosis VI cats. Mol. Ther. 2012, 20, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, T.T.; Maguire, A.M.; Aguirre, G.D.; Surace, E.M.; Anand, V.; Zeng, Y.; Salvetti, A.; Hopwood, J.J.; Haskins, M.E.; Bennett, J. Phenotypic rescue after adeno-associated virus-mediated delivery of 4-sulfatase to the retinal pigment epithelium of feline mucopolysaccharidosis VI. J. Gene Med. 2002, 4, 613–621. [Google Scholar] [CrossRef]
- Cotugno, G.; Annunziata, P.; Tessitore, A.; O’Malley, T.; Capalbo, A.; Faella, A.; Bartolomeo, R.; O’Donnell, P.; Wang, P.; Russo, F.; et al. Long-term amelioration of feline mucopolysaccharidosis VI after AAV-mediated liver gene transfer. Mol. Ther. 2011, 19, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferla, R.; O’Malley, T.; Calcedo, R.; O’Donnell, P.; Wang, P.; Cotugno, G.; Claudiani, P.; Wilson, J.M.; Haskins, M.; Auricchio, A. Gene therapy for mucopolysaccharidosis type VI Is effective in cats without pre-existing immunity to AAV8. Hum. Gene Ther. 2013, 24, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Ferla, R.; Alliegro, M.; Dell’Anno, M.; Nusco, E.; Cullen, J.M.; Smith, S.N.; Wolfsberg, T.G.; O’Donnell, P.; Wang, P.; Nguyen, A.-D.; et al. Low incidence of hepatocellular carcinoma in mice and cats treated with systemic adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2021, 20, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ferla, R.; Claudiani, P.; Cotugno, G.; Saccone, P.; De Leonibus, E.; Auricchio, A. Similar therapeutic efficacy between a single administration of gene therapy and multiple administrations of recombinant enzyme in a mouse model of lysosomal storage disease. Hum. Gene Ther. 2014, 25, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Ferla, R.; Alliegro, M.; Marteau, J.B.; Dell’Anno, M.; Nusco, E.; Pouillot, S.; Galimberti, S.; Valsecchi, M.G.; Zuliani, V.; Auricchio, A. Non-clinical Safety and Efficacy of an AAV2/8 Vector Administered Intravenously for Treatment of Mucopolysaccharidosis Type VI. Mol. Ther. Methods Clin. Dev. 2017, 6, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Byers, S.; Rothe, M.; Lalic, J.; Koldej, R.; Anson, D.S. Lentiviral-mediated correction of MPS VI cells and gene transfer to joint tissues. Mol. Genet. Metab. 2009, 97, 102–108. [Google Scholar] [CrossRef]
- Jackson, M.; Derrick Roberts, A.; Martin, E.; Rout-Pitt, N.; Gronthos, S.; Byers, S. Mucopolysaccharidosis enzyme production by bone marrow and dental pulp derived human mesenchymal stem cells. Mol. Genet. Metab. 2015, 114, 584–593. [Google Scholar] [CrossRef]
- Entchev, E.; Jantzen, I.; Masson, P.; Bocart, S.; Bournique, B.; Luccarini, J.M.; Bouchot, A.; Lacombe, O.; Junien, J.L.; Broqua, P.; et al. Odiparcil, a potential glycosaminoglycans clearance therapy in mucopolysaccharidosis VI—Evidence from in vitro and in vivo models. PLoS ONE 2020, 15, e0233032. [Google Scholar] [CrossRef]
- Entchev, E.; Antonelli, S.; Mauro, V.; Cimbolini, N.; Jantzen, I.; Roussey, A.; Germain, J.M.; Zhang, H.; Luccarrini, J.M.; Lacombe, O.; et al. MPS VI associated ocular phenotypes in an MPS VI murine model and the therapeutic effects of odiparcil treatment. Mol. Genet. Metab. 2021, S1096-7192. [Google Scholar] [CrossRef]
- Simonaro, C.M.; Ge, Y.; Eliyahu, E.; He, X.; Jepsen, K.J.; Schuchman, E.H. Involvement of the Toll-like receptor 4 pathway and use of TNF-α antagonists for treatment of the mucopolysaccharidoses. Proc. Natl. Acad. Sci. USA 2010, 107, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Schuchman, E.H.; Ge, Y.; Lai, A.; Borisov, Y.; Faillace, M.; Eliyahu, E.; He, X.; Iatridis, J.; Vlassara, H.; Striker, G.; et al. Pentosan Polysulfate: A Novel Therapy for the Mucopolysaccharidoses. PLoS ONE 2013, 8, e54459. [Google Scholar] [CrossRef] [Green Version]
- Frohbergh, M.; Ge, Y.; Meng, F.; Karabul, N.; Solyom, A.; Lai, A.; Iatridis, J.; Schuchman, E.H.; Simonaro, C.M. Dose responsive effects of subcutaneous pentosan polysulfate injection in mucopolysaccharidosis type VI rats and comparison to oral treatment. PLoS ONE 2014, 9, e100882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Grau, M.; Garrido, E.; Cozar, M.; Rodriguez-Sureda, V.; Domínguez, C.; Arenas, C.; Gatti, R.A.; Cormand, B.; Grinberg, D.; Vilageliu, L. Evaluation of aminoglycoside and non-aminoglycoside compounds for stop-codon readthrough therapy in four lysosomal storage diseases. PLoS ONE 2015, 10, e0135873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolomeo, R.; Polishchuk, E.V.; Volpi, N.; Polishchuk, R.S.; Auricchio, A. Pharmacological read-through of nonsense ARSB mutations as a potential therapeutic approach for mucopolysaccharidosis VI. J. Inherit. Metab. Dis. 2013, 36, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Chandler, R.J.; Sands, M.S.; Venditti, C.P. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum. Gene Ther. 2017, 28, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Alliegro, M.; Ferla, R.; Nusco, E.; De Leonibus, C.; Settembre, C.; Auricchio, A. Low-dose gene therapy reduces the frequency of enzyme replacement therapy in a mouse model of lysosomal storage disease. Mol. Ther. 2016, 24, 2054–2063. [Google Scholar] [CrossRef] [Green Version]
- Eliyahu, E.; Wolfson, T.; Ge, Y.; Jepsen, K.J.; Schuchman, E.H.; Simonaro, C.M. Anti-TNF-alpha therapy enhances the effects of enzyme replacement therapy in rats with mucopolysaccharidosis type VI. PLoS ONE 2011, 6, e22447. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.F.; Cantz, M.; Spranger, J. Maroteaux-Lamy disease (mucopolysaccharidosis VI), subtype A: Deficiency of a N-acetylgalactosamine-4-sulfatase. Biochem. Biophys. Res. Commun. 1974, 60, 1170–1177. [Google Scholar] [CrossRef]
- VIa, B.; Barer, F.S.; Pavlova, M.N.; Meerson, E.M. Ultrastructure of skin fibroblasts in storage diseases (mucopolysaccharidosis types IV and VI). Tsitol Genet. 1980, 14, 21–26. [Google Scholar]
- Bradford, T.M.; Litjens, T.; Parkinson, E.J.; Hopwood, J.J.; Brooks, D.A. Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome): A Y210C mutation causes either altered protein handling or altered protein function of N-acetylgalactosamine 4-sulfatase at multiple points in the vacuolar network. Biochemistry 2002, 41, 4962–4971. [Google Scholar] [CrossRef]
- Klein, U.; von Figura, K. Characterization of dermatan sulfate in mucopolysaccharidosis VI. Evidence for the absence of hyaluronidase-like enzymes in human skin fibroblasts. Biochim. Biophys. Acta 1980, 630, 10–14. [Google Scholar] [CrossRef]
- Steckel, F.; Hasilik, A.; von Figura, K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J. Biol Chem. 1983, 258, 14322–14326. [Google Scholar] [CrossRef]
- Pilz, H.; von Figura, K.; Goebel, H.H. Deficiency of arylsulfatase B in 2 brothers aged 40 and 38 years (Maroteaux-Lamy syndrome, type B). Ann. Neurol. 1979, 6, 315–325. [Google Scholar] [CrossRef]
- Alroy, J.; Freden, G.O.; Goyal, V.; Raghavan, S.S.; Schunk, K.L. Morphology of Leukocytes from Cats Affected with a-Mannosidosis and Mucopolysaccharidosis VI (MPS VI). Vet. Pathol. 1989, 26, 294–302. [Google Scholar] [CrossRef]
- Pohl, S.; Angermann, A.; Jeschke, A.; Hendrickx, G.; Yorgan, T.A.; Makrypidi-Fraune, G.; Steigert, A.; Kuehn, S.C.; Rolvien, T.; Schweizer, M.; et al. The Lysosomal Protein Arylsulfatase B Is a Key Enzyme Involved in Skeletal Turnover. J. Bone Miner. Res. 2018, 33, 2186–2201. [Google Scholar] [CrossRef] [Green Version]
- Golda, A.; Jurecka, A.; Gajda, K.; Tylki-Szymańska, A.; Lalik, A. Human pulmonary artery endothelial cells in the model of mucopolysaccharidosis VI present a prohypertensive phenotype. Mol. Genet. Metab. Rep. 2015, 3, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Pirozzi, M.; Auricchio, A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics 2009, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Evers, M.; Saftig, P.; Schmidtt, P.; Hafnert, A.; Mcloghlint, D.B.; Schmahlt, W.; Hess, B.; Von Figura, K.; Peters, C.; Neufeld, E.F. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis. Proc. Natl. Acad. Sci. USA 1996, 93, 8214–8219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtain, M.M.; Donahue, L.R. A Mutation in the Arsb Gene; A Mouse Model That Resembles Maroteaux_Lamy Syndrome. MGI Direct Data Submission MGI: J:149960. Available online: http://www.informatics.jax.org/reference/J:149960 (accessed on 3 September 2021).
- Wang, P.; Margolis, C.; Lin, G.; Buza, E.L.; Quick, S.; Raj, K.; Han, R.; Giger, U. Mucopolysaccharidosis Type VI in a Great Dane Caused by a Nonsense Mutation in the ARSB Gene. Vet. Pathol. 2018, 55, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskins, M.E. Animal models for mucopolysaccharidosis disorders and their clinical relevance. Acta Pædiatrica 2007, 96, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Simonaro, C.M.; Schuchman, E.H.; Ge, Y.; Laudier, D.M.; Iatridis, J.C. Structural, compositional, and biomechanical alterations of the lumbar spine in rats with mucopolysaccharidosis type VI (Maroteaux–Lamy syndrome). J. Orthop. Res. 2013, 31, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Noguchi, J.; Ikadai, H.; Takahashi, M.; Nagase, S. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J. Clin. Investig. 1993, 91, 1099–1104. [Google Scholar] [CrossRef]
- Jolly, R.D.; Hopwood, J.J.; Marshall, N.R.; Jenkins, K.S.; Thompson, D.J.; Dittmer, K.E.; Thompson, J.C.; Fedele, A.O.; Raj, K.; Giger, U. Mucopolysaccharidosis type VI in a Miniature Poodle-type dog caused by a deletion in the arylsulphatase B gene. N. Z. Vet. J. 2012, 60, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.L.; Kridel, H.A.; Gallagher, A.; Sheppard, B.J.; Reese, S.; Kondo, H.; Alleman, R.; Giger, U. Mucopolysaccharidosis type VI in a juvenile miniature schnauzer dog with concurrent hypertriglyceridemia, necrotizing pancreatitis, and diabetic ketoacidosis. Can. Vet. J. 2015, 56, 272–277. [Google Scholar] [PubMed]
- Haskins, M.E.; Jezyk, P.F.; Desnick, R.J.; Patterson, D.F. Mucopolysaccharidosis VI Maroteaux-Lamy syndrome. Arylsulfatase B-deficient mucopolysaccharidosis in the Siamese cat. Am. J. Pathol. 1981, 105, 191–193. [Google Scholar]
- Haskins, M.E.; Jezyk, P.F.; Patterson, D.F. Mucopolysaccharide storage disease in three families of cats with arylsulfatase B deficiency: Leukocyte studies and carrier identification. Pediatr. Res. 1979, 13, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Crawley, A.C.; Muller, V.J.; Hopwood, J.J. Two mutations within a feline mucopolysaccharidosis type VI colony cause three different clinical phenotypes. J. Clin. Investig. 1998, 101, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vine, D.T.; McGovern, M.M.; Haskins, M.E.; Desnick, R.J. Feline mucopolysaccharidosis VI: Purification and characterization of the residual arylsulfatase B activity. Am. J. Hum. Genet. 1981, 33, 916–927. [Google Scholar]
- Norrdin, R.W.; Moffat, K.S.; Thrall, M.A.; Gasper, P.W. Characterization of osteopenia in feline mucopolysaccharidosis VI and evaluation of bone marrow transplantation therapy. Bone 1993, 14, 361–367. [Google Scholar] [CrossRef]
- Dial, S.M.; Byrne, T.; Haskins, M.; Gasper, P.W.; Rose, B.; Wenger, D.A.; Thrall, M.A. Urine glycosaminoglycan concentrations in mucopolysaccharidosis VI-affected cats following bone marrow transplantation or leukocyte infusion. Clin. Chim. Acta 1997, 263, 1–14. [Google Scholar] [CrossRef]
- Lischka, F.W.; Gomez, G.; Yee, K.K.; Dankulich-Nagrundy, L.; Lo, L.; Haskins, M.E.; Rawson, N.E. Altered olfactory epithelial structure and function in feline models of mucopolysaccharidoses I and VI. J. Comp. Neurol. 2008, 511, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, J.D.; Brumfield, L.K.; Fazzalari, N.L.; Hopwood, J.J.; Byers, S. Histomorphometric analysis of the tibial growth plate in a feline model of mucopolysaccharidosis type VI. Calcif. Tissue Int. 1999, 65, 47–52. [Google Scholar] [CrossRef]
- Toyama, S.; Migita, O.; Fujino, M.; Kunieda, T.; Kosuga, M.; Fukuhara, Y.; Nagahara, Y.; Li, X.K.; Okuyama, T. Liver transplantation: New treatment for mucopolysaccharidosis type VI in rats. Pediatr. Int. 2019, 61, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Simonaro, C.M.; Haskins, M.E.; Kunieda, T.; Evans, S.M.; Visser, J.W.M.; Schuchman, E.H. Bone marrow transplantation in newborn rats with mucopolysaccharidosis type VI: Biochemical, pathological, and clinical findings. Transplantation 1997, 63, 1386–1393. [Google Scholar] [CrossRef]
- Yoshida, M.; Ikadai, H.; Maekawa, A.; Takahashi, M.; Nagaset, S. Pathological characteristics of mucopolysaccharidosis VI in the rat. J. Comp. Pathol. 1993, 109, 141–153. [Google Scholar] [CrossRef]
- Strauch, O.F.; Stypmann, J.; Reinheckel, T.; Martinez, E.; Haverkamp, W.; Peters, C. Cardiac and Ocular Pathologies in a Mouse Model of Mucopolysaccharidosis Type VI. Pediatr. Res. 2003, 54, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Kakkis, E.; Marsden, D. Urinary glycosaminoglycans as a potential biomarker for evaluating treatment efficacy in subjects with mucopolysaccharidoses. Mol. Genet. Metab. 2020, 130, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lehman, T.; Miller, N.; Norquist, B.; Underhill, L.; Keutzer, J. Diagnosis of the mucopolysaccharidoses. Rheumatology 2011, 50 (Suppl. 5), v41–v48. [Google Scholar] [CrossRef] [Green Version]
- Saville, J.T.; McDermott, B.K.; Fletcher, J.M.; Fuller, M. Disease and subtype specific signatures enable precise diagnosis of the mucopolysaccharidoses. Genet. Med. 2019, 21, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Langford-Smith, K.J.; Mercer, J.; Petty, J.; Tylee, K.; Church, H.; Roberts, J.; Moss, G.; Jones, S.; Wynn, R.; Wraith, J.E.; et al. Heparin cofactor II-thrombin complex and dermatan sulphate:chondroitin sulphate ratio are biomarkers of short- and long-term treatment effects in mucopolysaccharide diseases. J. Inherit. Metab. Dis. 2011, 34, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Raymond, G.V.; Pasquali, M.; Polgreen, L.E.; Dickson, P.I.; Miller, W.P.; Orchard, P.J.; Lund, T.C. Elevated cerebral spinal fluid biomarkers in children with mucopolysaccharidosis I-H. Sci. Rep. 2016, 6, 38305. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Vehe, R.K.; Rudser, K.; Kunin-Batson, A.; Utz, J.J.; Dickson, P.; Shapiro, E.; Whitley, C.B. Elevated TNF-α is associated with pain and physical disability in mucopolysaccharidosis types I., II, and VI. Mol. Genet. Metab. 2016, 117, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Heppner, J.M.; Zaucke, F.; Clarke, L.A. Extracellular matrix disruption is an early event in the pathogenesis of skeletal disease in mucopolysaccharidosis I. Mol. Genet. Metab. 2015, 114, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Batzios, S.P.; Zafeiriou, D.I.; Vargiami, E.; Karakiulakis, G.; Papakonstantinou, E. Differential expression of matrix metalloproteinases in the serum of patients with mucopolysaccharidoses. JIMD Rep. 2012, 3, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Mills, P.; Davison, J.; Cleary, M.; Gissen, P.; Banushi, B.; Doykov, I.; Dorman, M.; Mills, K.; Heywood, W.E. Free urinary glycosylated hydroxylysine as an indicator of altered collagen degradation in the mucopolysaccharidoses. J. Inherit. Metab. Dis. 2020, 43, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Heywood, W.; Camuzeau, S.; Doykov, I.; Patel, N.; Preece, R.; Footitt, E.; Cleary, M.; Clayton, P.; Grunewald, S.; Abulhoul, L.; et al. Proteomic Discovery and Development of a Multiplexed Targeted MRM-LC-MS/MS Assay for Urine Biomarkers of Extracellular Matrix Disruption in Mucopolysaccharidoses I, II, and VI. Anal. Chem. 2015, 87, 12238–12244. [Google Scholar] [CrossRef]
- Tebani, A.; Abily-Donval, L.; Schmitz-Afonso, I.; Piraud, M.; Ausseil, J.; Zerimech, F.; Pilon, C.; Pereira, T.; Marret, S.; Afonso, C.; et al. Analysis of mucopolysaccharidosis type VI through integrative functional metabolomics. Int. J. Mol. Sci. 2019, 20, 446. [Google Scholar] [CrossRef] [Green Version]

| Therapy | Description | In Vitro Studies | Animal Studies | Clinical Trials | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Ref | Model | Delivery Route | Ref | Phase | EudraCT Number | Clinical Trials.gov Identifier | ||
| Gene therapy | ARSB gene transfer mediated by retroviral vectors | feline muscle cells | [92] | cat | intravenous | [93] | - | - | - |
| ARSB gene transfer mediated by adeno-associated viral vectors | - | - | cat | subretinal | [94] | I/II | 2016-002328-10 | NCT03173521 | |
| cat | intravenous | [95,96,97] | |||||||
| mouse | intravenous | [97,98,99] | |||||||
| ARSB gene transfer mediated by lentiviral vectors | human fibroblasts, feline chondrocytes | [100] | rat | injection into the knee joint | [100] | - | - | - | |
| hHSCs, hBM-MSCs, hDP-MSCs | [101] | ||||||||
| Substrate reduction therapy | Odiparcil (substrate analogue for B4GalT7) | human fibroblasts | [102] | mouse | oral | [102,103] | II | 2017-002158-35 | NCT03370653 |
| Anti-inflammatory drugs | Infliximab (monoclonal antibody against human TNF-α) | - | - | rat | intravenous | [104] | - | - | - |
| Pentosan polysulfate | - | - | rat | oral | [105,106] | - | - | - | |
| rat | subcutaneous | [106] | |||||||
| Stop codon read-through | Gentamicin | human fibroblasts | [107,108] | - | - | - | - | - | - |
| PTC124 | human fibroblasts | [107,108] | - | - | - | - | - | - | |
| Year of Publication | Animal Model | Model Generation | Reference |
|---|---|---|---|
| 1977 | Siamese cat | Spontaneous | [64] |
| 1993 | Rat | Spontaneous | [127] |
| 1996 | Mouse | Targeted disruption | [122] |
| 2009 | Mouse | ENU mutagenesis | [123] |
| 2012 | Miniature Poodle dog | Spontaneous | [128] |
| 2015 | Miniature Schnauzer dog | Spontaneous | [129] |
| 2018 | Great Dane dog | Spontaneous | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Avanzo, F.; Zanetti, A.; De Filippis, C.; Tomanin, R. Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. Int. J. Mol. Sci. 2021, 22, 13456. https://doi.org/10.3390/ijms222413456
D’Avanzo F, Zanetti A, De Filippis C, Tomanin R. Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. International Journal of Molecular Sciences. 2021; 22(24):13456. https://doi.org/10.3390/ijms222413456
Chicago/Turabian StyleD’Avanzo, Francesca, Alessandra Zanetti, Concetta De Filippis, and Rosella Tomanin. 2021. "Mucopolysaccharidosis Type VI, an Updated Overview of the Disease" International Journal of Molecular Sciences 22, no. 24: 13456. https://doi.org/10.3390/ijms222413456
APA StyleD’Avanzo, F., Zanetti, A., De Filippis, C., & Tomanin, R. (2021). Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. International Journal of Molecular Sciences, 22(24), 13456. https://doi.org/10.3390/ijms222413456






